Abstract
The current study aimed to prepare the stable amorphous solid dispersions (ASD) and effervescence solid dispersions (ESD) of the poorly water-soluble Glibenclamide (GLB) and to enhance its solubility and physical stability. For this purpose, Kollidone VA 64, PEG-3350, and Gelucire 50/13 were selected as water-soluble carriers. The miscibility of the drug and carrier was predicted by molecular dynamics simulation studies, Hanson solubility parameters, Flory Huggins theory, and Gibb’s free energy. Solid dispersions were prepared by microwave, solvent evaporation, lyophilization, and Hot melt extruder (HME) methods. The prepared ASDs and ESDs were subjected to solubility and dissolution studies and other characterization studies. The in-silico and theoretical approach suggested that the selected polymers might exhibit better miscibility with the GLB. All solid dispersions had shown improved solubility and dissolution rate. But solid dispersions prepared with Kollidone VA64 and HME proved better solubility and dissolution. The solid-state characterizations like FTIR, $^{1}$H NMR proved the formation of intermolecular hydrogen bonding between the drug and carriers, which was comparatively more in ESDs than ASDs. Thermal analysis, PXRD, microscopic examination of GLB, ASDs, ad ESDs confirmed that the drug has converted to the amorphous form, which was more in ESDs than ASDs. Gibb’s free energy concept suggested that the prepared solid dispersions were stable at room temperature for 90 days. Ex-vivo intestinal absorption study on optimized ESDs designed by Kollidone VA64, HME technique exhibited higher flux and permeability coefficient when compared to pure drug suggesting better drug delivery. Amorphous solid dispersion and effervescence solid dispersions technology greatly enhanced Glibenclamide solubility, dissolution rate, and stability. Further, this might exhibit better bioavailability confirmed by an ex-vivo intestinal absorption study.
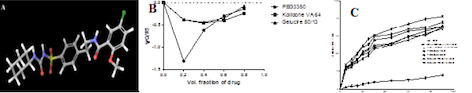
Fig. 3D-structure of GLB, Gibb’s free energy plot, and drug release plot
References
Li M, Gogos CG, Ioannidis N. (2015) Improving the API dissolution rate during pharmaceutical hot-melt extrusion I: Effect of the API particle size, and the co-rotating, twin-screw extruder screw configuration on the API dissolution rate. Int J Pharm 478(1):103-112. DOI: https://doi.org/10.1016/j.ijpharm.2014.11.024.
Figueirêdo CB, Nadvorny D, de Medeiros Vieira AC, de Medeiros Schver GC, Sobrinho JL, Neto PJ, Lee PI, Soares MF. (2018) Enhanced delivery of a fixed-dose combination of synergistic antichagasic agents posaconazole-benznidazole based on amorphous solid dispersions. Eur J Pharm Sci 119:208-218. DOI: https://doi.org/10.1016/j.ejps.2018.04.024.

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2022 Journal of Pharmaceutical Chemistry

